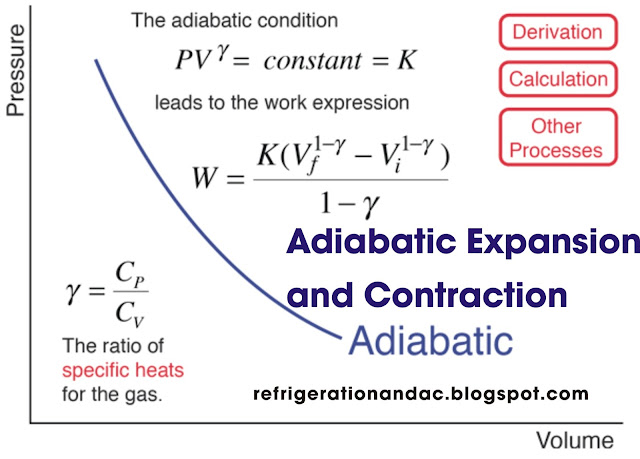
The term adiabatic refers to the process whereby a gas expands or contracts without any transfer of heat into it or from it during the expansion or compression. Adiabatic expansion and compression of gases would occur if the gas was placed in a perfectly insulated cylinder with a frictionless piston so that heat could not enter the gas during expansion or escape during compression.
During the adiabatic expansion of ideal gases, the work performed, compression and expansion are obtained FROM THE GAS. Thus, during the expansion, the pressure and temperature of the ideal gas decrease. Also, when work is done on gas as it is adiabatically compressed, the heat generated is not lost, but increases the temperature and consequently the pressure of the confined gas.
During the adiabatic expansion of ideal gases, the work performed, compression and expansion are obtained FROM THE GAS. Thus, during the expansion, the pressure and temperature of the ideal gas decrease. Also, when work is done on gas as it is adiabatically compressed, the heat generated is not lost, but increases the temperature and consequently the pressure of the confined gas.
Tags:
Basics of Refrigeration