In Fig. 1-19, refrigerant cylinder A is shown with the valve closed. The pressure, the temperature (inside and out), the number of molecules leaving the gaseous state, the number diving back into the liquid, and the liquid molecules flying out of the liquid into the gaseous state are equal. All conditions are balanced.
On cylinder B, the valve has been opened slightly and some of the gas is escaping. The results are twofold. The pressure over the liquid refrigerant in the cylinder is reduced. This will cause more of the liquid to change to a gas. In changing from a liquid to a gas, heat is absorbed and the liquid refrigerant will be cooled. The temperature of the refrigerant and cylinder is now 50 F. Some heat from the surrounding area, which is at 70 F., will now flow into the cylinder and into the refrigerant.
On cylinder C, the valve has been opened more than at B with the result that refrigerant gas flows out more rapidly. This results in still a lower pressure on the liquid refrigerant and more rapid evaporation of the refrigerant. This increase in the rate of evaporation lowers the temperature of the refrigerant and the cylinder still more with the result that the 72 F. air surrounding the cylinder will more rapidly give up its heat to the colder cylinder.
On cylinder A, we have a state of equilibrium with al I temperatures and pressures in balance.
On cylinder B, we have a slight unbalance due to the gas escaping through the valve. If this condition were to continue for a considerable time, a condition of balance would again prevail but in this case, its balance would not be a static one as in A; rather. a balance between the rate of heat flow into the cylinder, the evaporation of refrigerant, and the flow of refrigerant gas out of the cylinder valve. In this condition of balance, the refrigerant is cooling the cylinder and its surroundings.
On cylinder C, we have a greater unbalance than at cylinder B with the result that the cylinder pressure and temperature will be lowered still further.
As long as the valve is open and gas molecules can escape, the temperature will be lower because more liquid molecules are becoming gas molecules, then gas molecules are returning to the liquid. This gas bombardment is called vapor pressure. If this vapor pressure can be reduced, the temperature of the liquid can be reduced since evaporation will be increased.
If the gas molecules can be removed fast enough by any means such as suction from a compressor, a chemical to absorb the molecules, or other means, a low enough vapor pressure may be produced to create refrigerant boiling temperatures which are at the refrigerating level.
The operation of the mechanical refrigerator is based on the heat absorption property of a fluid passing from the liquid to the gaseous state. If one were to put a cylinder of refrigerant into an icebox and vent the gas to the outside, we would have a heat absorber in the box, as shown in Fig. 1-20. The liquid can boil only at its evaporation temperature, say 20 F., and this liquid will be at this temperature until it has completely evaporated. If one tried to raise its temperature by adding heat, the only result would be a more rapid evaporation of the liquid into a gas, provided the pressure remained constant. Being at this low temperature there is, of course, a transfer of heat to it from the surrounding objects. This heat helps the evaporating and the heat is carried away in the vapor passing off. Thus, the fluid changing its state to gas gets the energy (or heat) for doing this from the objects surrounding it, and that heat is removed with the vapor to the outside of the box.
This type of refrigerator works nicely, but it is an expensive method because the refrigerant fluid is lost. There are some mobile refrigerating units (trucks) that use this method. The refrigerant used is usually liquid nitrogen which is relatively inexpensive. It is called chemical refrigeration.
In the mechanical refrigerator, this escaping vapor is captured, compressed, and cooled to a liquid state again so that it can be used over and over, as shown in Fig. 1-21.
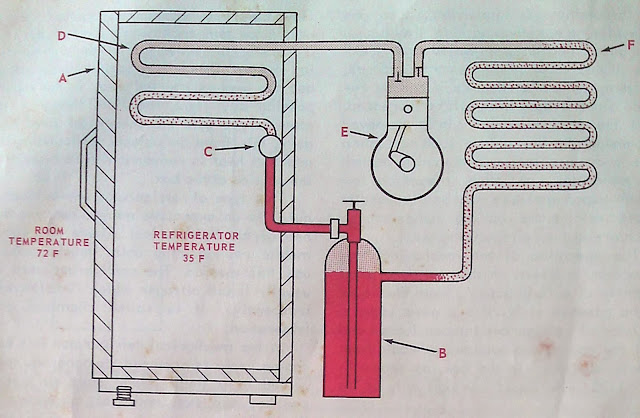
To follow through the refrigerant cycle in this refrigerator mechanism, begin with the refrigerant in tank B. The refrigerant used in this illustration is R-12.
The refrigerant at B is under a pressure corresponding to the room temperature of 72 F. For R-12 this pressure will be approximately 71 psi. At the refrigerant control, C, this pressure is reduced to provide low-pressure, low-temperature evaporation in the evaporating (cooling) coil. Since the refrigerant temperature is to be held at 35 F. the pressure in the evaporating coil must be held at or below 30 psi. It is the purpose of the refrigerant control to allow refrigerant to flow from the storage tank (high side) into the evaporator coil (low side) while at the same time maintaining the pressure difference between the high-pressure side (high side) and the low-pressure side (low side). In evaporator, D, the liquid refrigerant is now under a much-reduced pressure and it will evaporate or boil very rapidly thus cooling the evaporator coil.
 |
| |
The compressor, E, draws (sucks) the evaporated refrigerant gas from the evaporator coil and compresses it back to the high side pressure. From the compressor, the high temperature (see heat of compression, Par. 1-33) high-pressure gaseous refrigerant flows into the condenser coils F. The temperature of the gas as it enters these coils will be several degrees warmer than the room temperature. This results in a very rapid transfer of heat from the condenser coil to the surrounding air. The gas as it flows through the condenser is cooled and loses its heat of evaporation and returns to the liquid state again. As a liquid it flows from the condenser coils back into the liquid refrigerant storage at B. This refrigeration cycle is repeated over and over until the desired temperature is reached and a thermostat breaks the circuit to the driving motor and the compressor stops.
The temperature at which a refrigerator cooling unit is kept depends on the pressure at which the refrigerant is evaporated, while the amount of heat removed depends entirely on the amount of refrigerant changed into a gas.
Tags:
Basics of Refrigeration

